When Were Cannabinoids Discovered?

The discovery of cannabinoids has slowly revealed the cannabis plant to be a fountain of potential. Their identification even predates the discovery of the endocannabinoid system (ECS), and in fact was crucial to helping researchers uncover the physiological network.
Researchers have identified over 100 unique cannabinoids. Some of these molecules have changed the face of cannabis science, whereas others remain relatively enigmatic and untouched. Below, we'll take a look at when the most familiar cannabinoids were discovered, and who deserves credit for these groundbreaking findings.
Early pioneers of cannabinoid research
Before individual cannabinoids were isolated and identified, an early group of forerunner scientists laid the critical foundations for these discoveries. Investigators started achieving crude cannabis extracts in the early 19th century, which would soon evolve into capturing specific molecules.
In 1840, a researcher by the name of Schlesinger reportedly obtained the first active extract[1] from the flowers and leaves of hemp plants. Shortly after, another experimental mind named Decourtive conducted an ethanol extract of similar materials in 1848. He evaporated the alcohol and found a dark resin in its place. He named the substance “cannabin”.
Other researchers took it upon themselves to test the effects of this substance. Later in the 19th century, scientists prepared an alcohol extraction and added a lime solution to remove the chlorophyll. They went on to filter and treat the mixture with sulfuric acid, followed by evaporation. The researchers tested the leftover resin and found it to be of neutral pH. After trying “two-thirds of a grain” of the substance, they described its “powerful narcotic effects”.
Scientists were on the trail of a new compound, but they couldn’t quite figure out what was generating these effects. Many argued cannabis housed alkaloids, with Preobrajensky claiming the plant to possess nicotine.
In the hunt for alkaloids, Klein and colleagues isolated cannabimines A, B, C, and D. Shortly after, researchers isolated the alkaloids cannabisativine, from the roots of a Mexican landrace, and anhydrocannabisativine, from the roots and leaves of another wild Mexican strain.
Although these substances appeared to generate some effects in mice, it became clear that other compounds were responsible for the unique effects of the cannabis plant. Soon, scientists would stumble across a unique chemical family that underpinned these properties: cannabinoids.
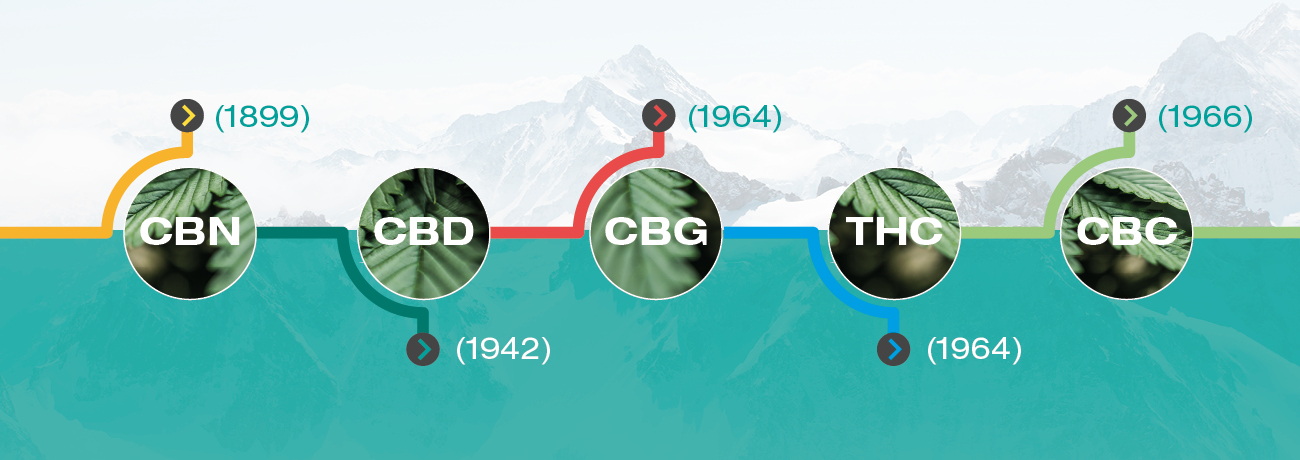
• CBN (1899)
CBN, or cannabinol, was the first cannabinoid to be isolated from the cannabis plant. Thomas Wood, W.T. Spivey, and Thomas Easterfield made this groundbreaking discovery[2] in 1899. They obtained the molecule from a sample of charas, a hand-rolled form of cannabis resin. After making an ethanol extract from the sample, they ran it through fractional distillation and produced a viscous oil.
They described the result of this process as “amber-coloured when seen in thin layers but ruby red when seen in mass”. They found the substance to possess psychoactive effects in doses as low as 0.05g. After conducting acetylation, they discovered CBN.
However, cannabis plants don’t create CBN through enzymatic processes. Instead, the cannabinoid results from the degradation of THC. Therefore, the research team may have been working with old samples of extracts in which the THC had broken down.
• CBD (1942)
United States chemist Roger Adams first isolated CBD[3] (cannabidiol) from cannabis in 1942. He extracted the now world-famous cannabinoid from the flowers of Minnesota wild hemp. Adams conducted an ethanol extraction to create a “red oil”. He then ran the substance through distillation under diminished pressure and isolated cannabidiol from the mix of constituents.
Decades later, in 1963, researchers made another important discovery regarding CBD—the molecular structure of the cannabinoid. Raphael Mechoulam, organic chemist and professor at the Hebrew University of Jerusalem, made this important finding[4].
• CBG (1964)
CBG (cannabigerol) stems from cannabinoid acid CBGA, which plays an integral role in cannabinoid biosynthesis. Many cannabinoids that derive from enzymatic reactions start off life as CBGA, making it somewhat of a master precursor. Several enzymes act on this cannabinoid acid and convert it into other members of the cannabinoid family. For example, CBDA synthase converts CBGA into CBDA, which then decarboxylates into CBD when heated.
Raphael Mechoulam and Yechiel Gaoni joined forces in 1963 to work on cannabis research for the next four years. This pairing would result in a spree of pioneering discoveries in the world of cannabis science. In 1964, the pair set about investigating the pathways of cannabinoid biosynthesis in the plant. They soon solved the problem of a missing link: Where were all of these cannabinoids coming from? They identified CBGA[5] as the bridge between other chemicals becoming cannabinoids.
• THC (1964)
THC produces most of the psychoactive effects associated with cannabis. This controversial molecule attaches to the CB1 receptor of the endocannabinoid system to produce these outcomes. Shortly after discovering CBG, Mechoulam and Gaoni cracked the code and identified and isolated THC[6] (delta-9-tetrahydrocannabinol).
The duo went on to synthesise THC for the first time in 1965 and then again in 1967. Despite their immense success, Mechoulam attributes their discoveries to cannabis science conducted earlier in the century. Although they officially isolated THC during 1964, other scientists laid the groundwork for this discovery.
Both Roger Adams and Alex Todd managed to synthesise molecules extremely close in structure to THC in the 1940s. These researchers never managed to isolate the cannabinoid, but used cannabinoids such as CBD to create similar molecules. Mechoulam states they were likely limited by the literature and techniques available to them at the time.
• CBC (1966)
CBC (cannabichromene) makes up around 0.3% of cannabis plant extract. However, breeders have developed cultivars expressing significantly higher amounts. A non-psychoactive cannabinoid, researchers have explored the pharmacological actions[7] of CBC, finding some intriguing potential.
Interestingly, two different teams identified CBC during the same year, using two different methods. Mechoulam and Gaoni isolated and identified the structure of CBC[8] in 1966. Starting out with a hexane extract, the two researchers ran a chromatography test and identified CBD, THC, CBN, CBG, and CBC. Cannabichromene made up around 1.5% of the extract.
They took the fractions containing CBC and re-chromatographed them twice before distillation. Using this process, they isolated CBC and proceeded to identify the molecular structure of the cannabinoid. Reports also state that a team of German researchers—Claussen, Von Spulak, and Korte—managed to isolate CBC during the same year using benzene percolation of hemp.
Cannabinoids: The tip of the iceberg
The five molecules above make up the major cannabinoids found in cannabis plants. Scientists have probed these chemicals relatively in-depth. However, a host of over 100 cannabinoids and cannabinoid acids are waiting to be fully elucidated by researchers. The next few decades will surely see massive steps forward in the field of cannabis science.
[1] Mechoulam, R., & Hanuš, L. (2000, June). A historical overview of chemical research on cannabinoids. https://1d7u564dod7i2q96m533sblk-wpengine.netdna-ssl.com/wp-content/uploads/2018/08/A-historical-overview-of-chemical-research-on-cannabinoids.pdf [Source]
[2] Wood, T. B., Spivey, W. T. N., & Easterfield, T. H. (1899). III.—Cannabinol. Part I. J. Chem. Soc., Trans., 75(0), 20–36. https://doi.org/10.1039/ct8997500020 [Source]
[3] Adams, R., Hunt, M., & Clark, J. H. (1940). Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. Journal of the American Chemical Society, 62(1), 196–200. https://doi.org/10.1021/ja01858a058 [Source]
[4] Mechoulam, R., & Shvo, Y. (1963). Hashish—I. Tetrahedron, 19(12), 2073–2078. https://doi.org/10.1016/0040-4020(63)85022-x [Source]
[5] Gaoni, Y., & Mechoulam, R. (1964). Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. Journal of the American Chemical Society, 86(8), 1646–1647. https://doi.org/10.1021/ja01062a046 [Source]
[6] Mechoulam, R. (1986). Interview with Prof. Raphael Mechoulam, Codiscoverer of THC. International Journal of the Addictions, 21(4–5), 579–587. https://doi.org/10.3109/10826088609083542 [Source]
[7] Russo, E. B., & Marcu, J. (2017). Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Cannabinoid Pharmacology, 67–134. https://doi.org/10.1016/bs.apha.2017.03.004 [Source]
[8] Gaoni, Y., & Mechoulam, R. (1966). Cannabichromene, a new active principle in hashish. Chemical Communications (London), 1, 20. https://doi.org/10.1039/c19660000020 [Source]
[1] Mechoulam, R., & Hanuš, L. (2000, June). A historical overview of chemical research on cannabinoids. https://1d7u564dod7i2q96m533sblk-wpengine.netdna-ssl.com/wp-content/uploads/2018/08/A-historical-overview-of-chemical-research-on-cannabinoids.pdf [Source]
[2] Wood, T. B., Spivey, W. T. N., & Easterfield, T. H. (1899). III.—Cannabinol. Part I. J. Chem. Soc., Trans., 75(0), 20–36. https://doi.org/10.1039/ct8997500020 [Source]
[3] Adams, R., Hunt, M., & Clark, J. H. (1940). Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. Journal of the American Chemical Society, 62(1), 196–200. https://doi.org/10.1021/ja01858a058 [Source]
[4] Mechoulam, R., & Shvo, Y. (1963). Hashish—I. Tetrahedron, 19(12), 2073–2078. https://doi.org/10.1016/0040-4020(63)85022-x [Source]
[5] Gaoni, Y., & Mechoulam, R. (1964). Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. Journal of the American Chemical Society, 86(8), 1646–1647. https://doi.org/10.1021/ja01062a046 [Source]
[6] Mechoulam, R. (1986). Interview with Prof. Raphael Mechoulam, Codiscoverer of THC. International Journal of the Addictions, 21(4–5), 579–587. https://doi.org/10.3109/10826088609083542 [Source]
[7] Russo, E. B., & Marcu, J. (2017). Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Cannabinoid Pharmacology, 67–134. https://doi.org/10.1016/bs.apha.2017.03.004 [Source]
[8] Gaoni, Y., & Mechoulam, R. (1966). Cannabichromene, a new active principle in hashish. Chemical Communications (London), 1, 20. https://doi.org/10.1039/c19660000020 [Source]







