How are cannabinoids produced in the cannabis plant?

Cannabinoids are a naturally occurring class of chemicals that interface with the endocannabinoid system—a regulatory network that governs homeostasis in the human body. Researchers have studied many of these molecules for their therapeutic potential.
Contents:
What are cannabinoids?
The cannabis plant produces over 100 unique cannabinoids. THC and CBD are the most well-known among them, with CBC, THCV, CBDV, CBG, and CBN making up the rest of the major cannabinoids. All of these molecules occur at varying levels in different cultivars. Cannabinoids also occur elsewhere in the plant kingdom. Hops, rosemary, black pepper, cloves, kava, and lemon balm all produce cannabinoids.
How are cannabinoids produced?
Cannabinoid production takes place in small glands known as trichomes. These crystalline structures occur in the highest density on cannabis flowers, and in lesser numbers on leaves, stems, and other aerial parts. Trichomes are essentially minute chemical factories. They are tasked with churning out secondary metabolites—cannabinoids, terpenes, and flavonoids—to protect the plant against pests, defend against extreme temperatures, and attract pollinators.
Female plants produce more cannabinoids than their male counterparts and possess three different types of trichomes: bulbous, capitate sessile, and capitate-stalked. The latter produce the most cannabinoids out of the trio. These structures feature a round sphere at the end of a stem-like base.
The complex process of cannabinoid production—or cannabinoid biosynthesis—takes place in the intricate cuticle. Cannabinoids are formed in specialised disk cells within this structure, before being ejected through the secretory cavity onto the surface of the plant in the form of a viscous resin.
Understanding cannabinoid biosynthesis
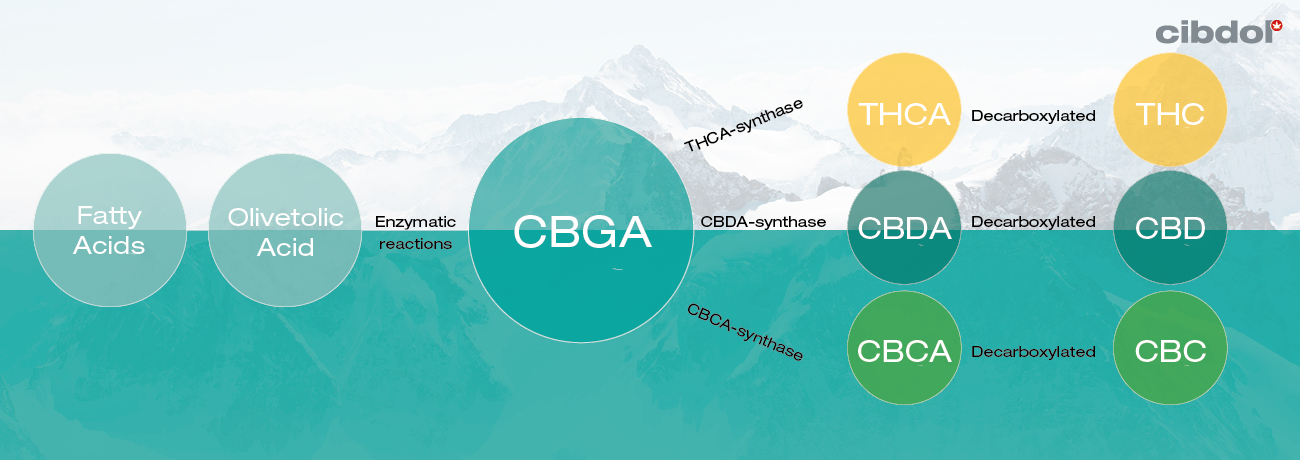
Cannabinoid biosynthesis starts out with the fatty acid called hexanoic acid. From here, enzymes facilitate a chemical reaction that causes phenolic precursors to form, which are then turned into olivetolic acid and geranyl pyrophosphate. These two molecules are fused together via another enzymatic reaction to produce the so-called “mother cannabinoid” CBGA.
This molecule—along with three other enzymes—is involved in the creation of all other cannabinoids that require enzymatic modification in their pathway. The enzymes THCA synthase, CBDA synthase, and CBCA synthase convert CBGA into THCA, CBDA, and CBCA, respectively.
These so-called cannabinoid acids have some uses of their own, but their “activated” versions are more sought-after. The “A” within these chemical names signifies a carboxyl group, which is ejected from each molecule via decarboxylation. For example, THCA converts to THC, and so forth. Decarboxylation can occur instantly via heat, or it can happen over time through exposure to the elements.
Specific cannabinoids have the potential to give rise to other cannabinoids when exposed to certain environmental factors. For instance, UV light can convert CBCA into CBLA, and oxidation can turn THC into CBN, another psychoactive cannabinoid.
In summary:
• Enzymatic reactions turn olivetolic acid and geranyl pyrophosphate into CBGA.
• THCA synthase, CBDA synthase, and CBCA synthase convert CBGA into THCA, CBDA, and CBCA.
• CBDA, THCA, CBCA, and other cannabinoids are decarboxylated into CBD, THC, CBC…
• Oxidation and exposure to the elements causes some new cannabinoids, such as CBN, to form.
The complex world of cannabinoids
The process of cannabinoid biosynthesis is worthy of a much longer, more in-depth text, but we hope to have provided some clarity on a complex topic. Understanding the series of chemical reactions that lead to the creation of various cannabinoids will undoubtedly lead to a greater understanding of cannabinoids in general.